Abstract
BACKGROUND
Lenalidomide, bortezomib, and dexamethasone (RVd) is the most common induction regimen for patients with NDMM before ASCT. In 2017, lenalidomide (R), as maintenance therapy (R-MT), became the first approved drug for patients (pts) who had undergone an upfront ASCT in the United States (US) and Europe. While other therapies (eg, thalidomide, bortezomib) have been studied in the maintenance setting they are not currently approved or proven to improve survival. There is limited real-world evidence comparing treatment (Tx) outcomes in pts who receive RVd followed by ASCT and R-MT vs those who receive RVd induction followed by ASCT and other maintenance therapies (O-MT) or no maintenance therapy (No-MT). This study aimed to assess real-world outcomes associated with these different sequencing approaches.
METHODS
A retrospective observational study of pts with NDMM was conducted using electronic health records from a nationally representative database (Flatiron Health). The Flatiron MM provider network comprises over 265 clinics throughout the US. Pts with an ICD-9 (203.0x) or ICD-10 (C90.xx) diagnosis of MM between 01/01/2011 and 05/31/2017 were randomly selected into the study. Pts were excluded if they did not have ≥ 2 documented clinical visits during the study period. The diagnosis of MM was confirmed through review of unstructured chart data (eg, physician notes). The index date was defined as the earlier of date of ASCT + 90 days or start of MT. Pts with NDMM were defined as those without a MM Tx > 14 days prior to their first diagnosis date. The start of first-line (1L) therapy was defined as the first episode of an eligible systemic Tx given after or up to 14 days before the MM diagnosis. Regimens were defined using the first eligible drug episode plus other eligible drugs given within 28 days of each other. A maximum gap of 180 days was allowed within a given line of therapy (LOT). Clinical characteristics at baseline were assessed at diagnosis as well as the index date. Treatments not considered consolidation or MT were considered as the next LOT. Time to next treatment (TTNT) was defined as the duration from the index date until the initiation of another LOT. The Kaplan-Meier (KM) method was used to calculate median TTNT, and the Cox proportional hazards model was used to evaluate risk factors for TTNT.
RESULTS
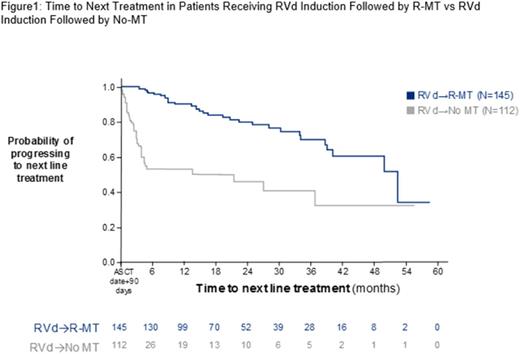
Of 632 patients receiving ASCT in the first LOT, 280 pts (44.3%) received RVd induction and met inclusion criteria. Among these, 145 received R-MT, 112 received No-MT, and 23 received O-MT. Sample sizes limited statistical comparisons between the R-MT and O-MT groups. There were no significant differences in age (59.7 vs 59.6 years), sex, race, geographical region, or practice type between R-MT and No-MT pts. A smaller proportion of R-MT pts progressed to a second line (2L) than No-MT pts (22.1% vs 34.1%; P= .004). The proportion of pts receiving O-MT who received a 2L was 47.8%. Second line regimens varied in frequency in each MT group and included Rd, RVd, Vd, and other triplet combinations. Pts with R-MT had a significantly longer median TTNT (52.24 months [95% CI, 39.97-NR]) than pts with No-MT (21.32 months [95% CI, 3.82 months-NR]; P < .001); Figure 1). Factors associated with a higher probability of advancing to 2L included No-MT (HR, 5.466 [95% CI, 3.34-8.946]; P < .001) and presence of anemia at baseline (HR, 1.65 [95% CI, 1.322-2.059]; P < .0001).
CONCLUSION
For pts with NDMM who are eligible for ASCT, R-MT was the most frequent maintenance therapy in pts receiving first-line RVd. The regimen of RVd induction followed by R-MT had superior outcomes compared with No-MT. Pts on R-MT were less likely to progress and had a significantly longer TTNT than No-MT pts. In the real-world setting, these results appear to be similar to those observed in clinical trials (McCarthy et al. N Engl J Med . 2012; McCarthy PL. J Clin Oncol . 2017). A great level of heterogeneity existed among second line regimens. Future studies should be conducted to better understand patient and disease characteristics that are predictors of receiving specific maintenance and second line therapies.
Fonseca: Celgene Corporation: Consultancy, Research Funding; Jansen: Consultancy; Mayo Clinic & Dr Fonseca: Patents & Royalties: Prognostication of myeloma via FISH, ~$2000/year; Novartis: Consultancy; Takeda: Consultancy; AMGEN: Consultancy; Bayer: Consultancy; Pharmacyclics: Consultancy; Merck: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Bristol-Myers Squibb: Consultancy. Parikh: Celgene Corporation: Employment. Ung: Celgene Corporation: Other: Fellowship is funded by Celgene. Ni: Celgene: Employment. Abouzaid: Celgene Corporation: Employment, Equity Ownership, Research Funding. Agarwal: Celgene Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal